Gene Therapy for Alzheimer's Disease and Ethical Aspects of Genome Editing (2020-2021)
This project team examined the causative factors and the mechanisms behind the onset of Late-onset Alzheimer’s Disease (LOAD) in order to advance the research and development of new gene therapy technologies applicable to treating age-related brain diseases.
Team members designed an experimental plan and participated in experiments to test the hypothesis that the association of the APOE gene with LOAD is mediated by dysregulation of APOE expression. The team focused on modulating APOE expression levels as a therapeutic target for LOAD using innovative genome and epigenome editing tools based on CRISPR/Cas9 technologies. The outcomes of this research will greatly facilitate the development of “smart” drugs for treating LOAD.
At the same time, team members investigated the ethical, legal and social questions related to the CRISPR/Cas9 technology and gene therapy for LOAD and other late-onset neurodegenerative conditions. Team members undertook case studies of earlier gene therapies and traced their development; evaluated the ethical issues associated with the use of gene editing technologies; analyzed novel questions of cost and pricing for so-called “one and done” therapies; and mined the literature for approaches to late-onset diseases that have reckoned with both treatment and quality-of-life issues.
Timing
Summer 2020 – Spring 2021
Team Outputs
Molecular Tools to Address Alzheimer's Disease (2021 Fortin Foundation Bass Connections Virtual Showcase)
Gene Therapy for Alzheimer's Disease and Ethical Aspects of Genome Editing (poster by Angela Wei, Anna Yang, Ishika Gupta, Mohanapriya Cumaran, Natalie Asmus, Sahil Malhotra, Suraj Upadhya, Boris Kantor, Misha Angrist and Ornit Chiba-Falek)
Treating Alzheimer’s with Gene Therapy (lightning talk by Ishika Gupta and Sahil Malhotra, April 2021)
Reflections
This Team in the News
Senior Spotlight: Reflections from the Class of 2023
See related teams, Treating Alzheimer's with Gene Therapy and the Ethical, Legal and Social Implications (2021-2022) and Gene Therapy in Alzheimer’s Disease: Novel Therapies and Ethical Aspects of Somatic Gene Editing (2019-2020).
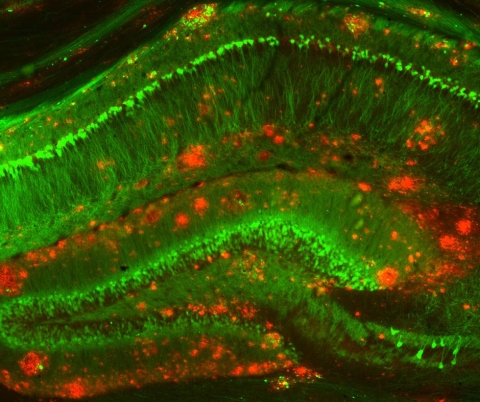
Image: Mouse model of Alzheimer's disease, by NIH Image Gallery, licensed under CC BY-NC 2.0
Team Leaders
- Misha Angrist, Social Science Research Institute
- Ornit Chiba-Falek, School of Medicine-Neurology
- Boris Kantor, School of Medicine-Neurobiology
/undergraduate Team Members
-
Natalie Asmus, Biology (BS)
-
Mohanapriya Cumaran, Biomedical Engineering (BSE)
-
Ishika Gupta, Psychology (BS)
-
Sahil Malhotra, Neuroscience (BS)
-
Suraj Upadhya, Biomedical Engineering (BSE)
-
Angela Wei, Biology (BS)
-
Anna Yang, Biology (BS)
/yfaculty/staff Team Members
-
Julio Barrera, School of Medicine-Neurology
-
Logan Brown, School of Medicine-Neurobiology
